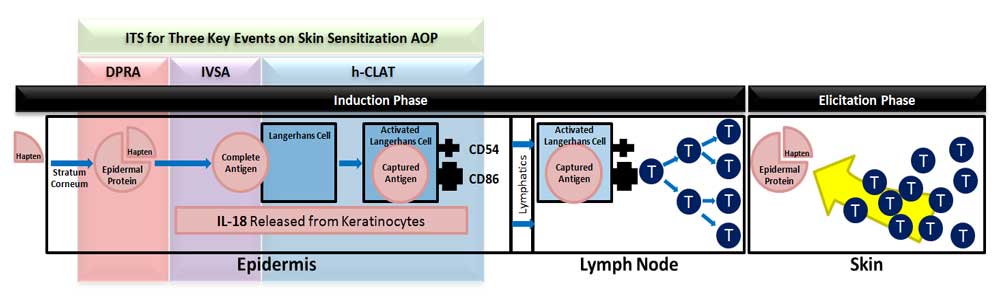
MB Research has developed a non-animal Integrated In Vitro Sensitzation (IIVS) testing strategy for chemical-induced contact hypersensitivity (skin sensitization). The IIVS provides a completely non-animal set of in vitro assays to replace in vivo test methods like the Murine LLNA or guinea pig based tests. When combined, the three IIVS assays address key events on the skin sensitization adverse outcome pathway (AOP).
The In Vitro Sensitization Assay (IVSA) is a keratinocyte activation test. IVSA uses an ELISA method to measure IL-18 release from a topically treated reconstructed 3D human keratinocyte tissue model.
The Human Cell Line Activation Test (h-CLAT) is a dendritic cell activation test. The h-CLAT uses flow cytometry to measure CD86 and CD54 expression on treated THP-1 cells in culture.
The Direct Peptide Reactivity Assay (DPRA) is an in chemico method used to predict epidermal protein binding. Binding of epidermal proteins is the molecular initiating event on the AOP. The DPRA uses HPLC to measure the depletion of synthetic peptides in solution following exposure to test chemicals.